HOME / SCIENCE / RAS pathway and cancer
RAS pathway and cancer

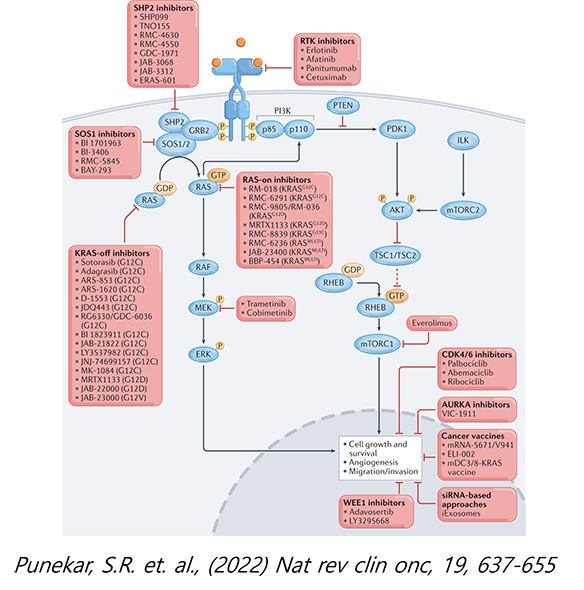
RAS GTPase family has 3 members encoding KRAS, HRAS and NRAS proteins which are involved in controlling normal growth signal. RAS GTPases are also well-known oncogenes that drive tumorigenesis when mutation is acquired. RAS oncogene mutations have been documented in more than 25% of total cancer patients worldwide and in cancer patients of Lung, Colon and Pancreas with high frequencies, especially. There were many attempts to find the effective drug to treat cancer patients with RAS mutation for last 40 years without success. Consequently, RAS protein was considered as an undruggable target. However, impressive clinical responses were observed in trial of Lumakras (sotorasib, Amgen) and Kraziti (adagrasib, Mirati) utilizing compound’s ability to form covalent bond with cysteine specifically in KRAS G12C mutated cancer (14% of KRAS mutated cancers). The successful approval of Lumakras is heightened hope and anticipation of developing therapeutics to treat KRAS mutated cancer.
At Txinno Bioscience, we are developing “ULK1” inhibitor (best-in-class), “Target S” inhibitor (best-in-class) and “Target Y“ inhibitor (first-in-class) to treat cancer patients with KRAS mutations.
ULK1 inhibitor/TPD utilizing synthetic lethality to target KRAS mutated cancers
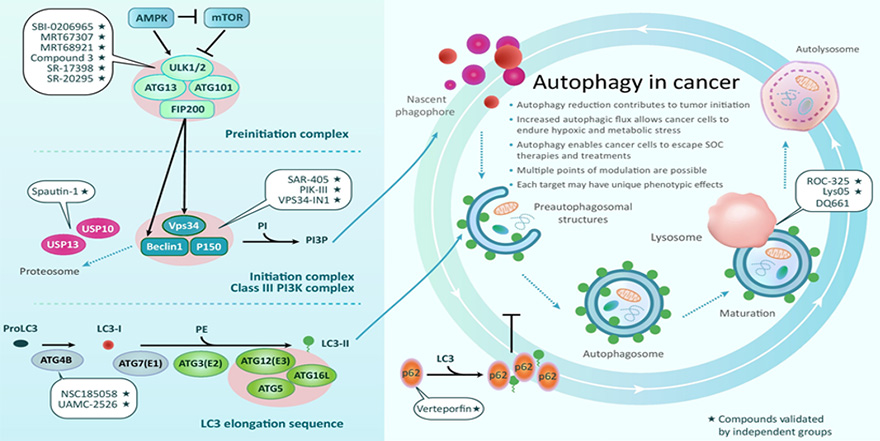
ULK1 is a serine/threonine protein kinase that involved in initiating autophagy process when cells encounter stressful conditions, like starvation or oxidative assaults. Autophagy has been known to play important roles in survival, drug resistance as well as immune avoidance of cancer cells. Interestingly, inhibition of ULK1 has been reported to enhance the efficacy of MEK kinase inhibitor when KRAS-mutated cancer cells were subjected to inhibition of both kinases simultaneously (Bryan D. Smith, et. al. (2019) AACR-EORTC poster presentation).
At Txinno Bioscience, we are developing ULK1 ULK1 inhibitor/TPD (combination of KRAS G12C or EGFR inhibitor) as a synthetic lethality drug for KRAS or EGFR mutant cancers. This combination therapy 1) can be applied to cancers with mutations other than KRAS G12C mutations, 2) can overcome EGFR treatment resistance, and 3) can be expected to improve therapeutic efficacy at the same or lower doses of existing targeted anticancer drugs when used in combination.
Inhibitors of mutated KRAS
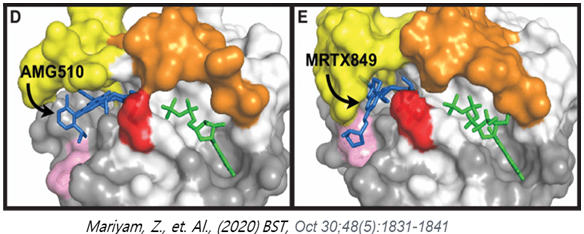
Recently, Lumakras and Krazati which are KRAS G12C inhibitors forming covalent bond with mutated KRAS protein were approved by FDA and are leading a great advancement of treating non small cell lung cancer patients. However, because the approved G12C inhibitors exert their inhibitory activities by forming covalent bond with cysteine residue only found in 14% patients among all KRAS mutated cancer patients, there is a significant limitation (lack of benefit) against other KRAS mutations which occurs in rest of 85% KRAS mutation cancer patients. Also, recent publication showed that about 30% of KRAS G12C cancer patients treated with G12C inhibitor (Adagrasib, Mirati) eventually developed drug resistance acquired by additional mutation other than G12C. There is a tremendous demands on new drug against KRAS mutated cancer harboring mutations other than G12C.
At Txinno Bioscience, we are developing small molecule ’Target S’ inhibitor that directly inhibit mutant KRAS protein. These inhibitors has advantages of treating larger population of KRAS mutated cancer patients than that of KRAS G12C carrying patients.
